Designer Toxins Intro
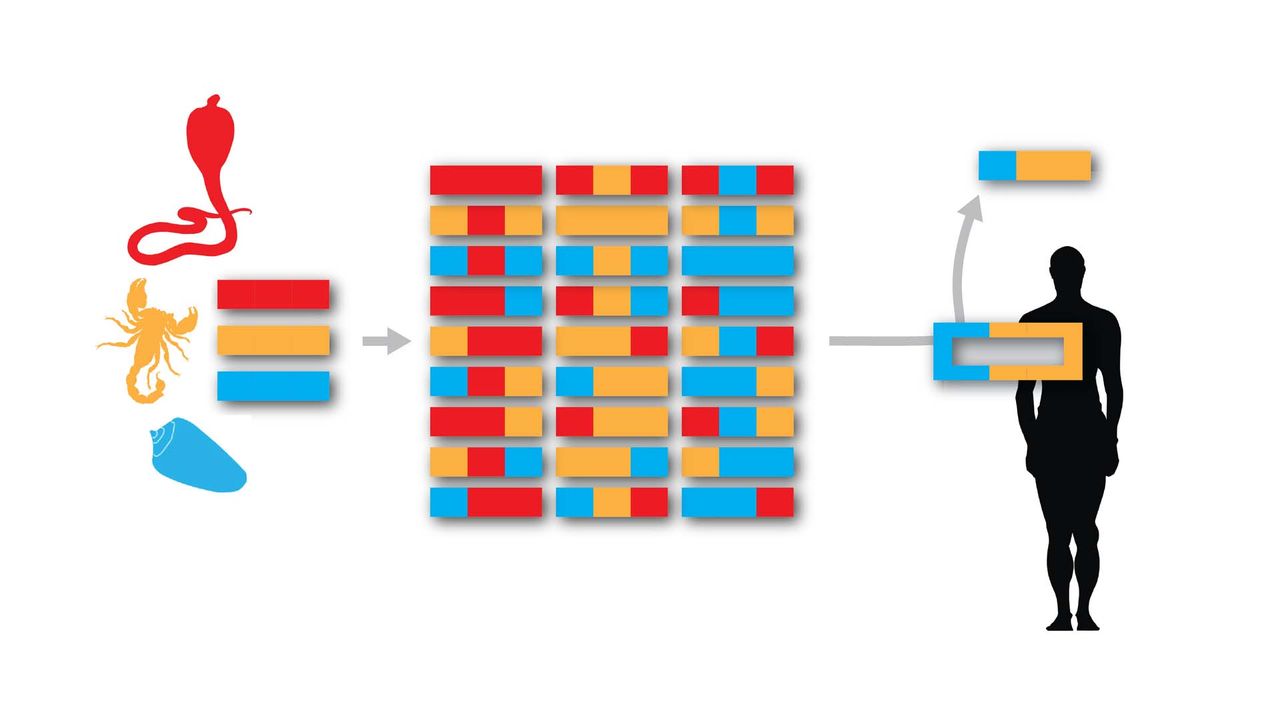
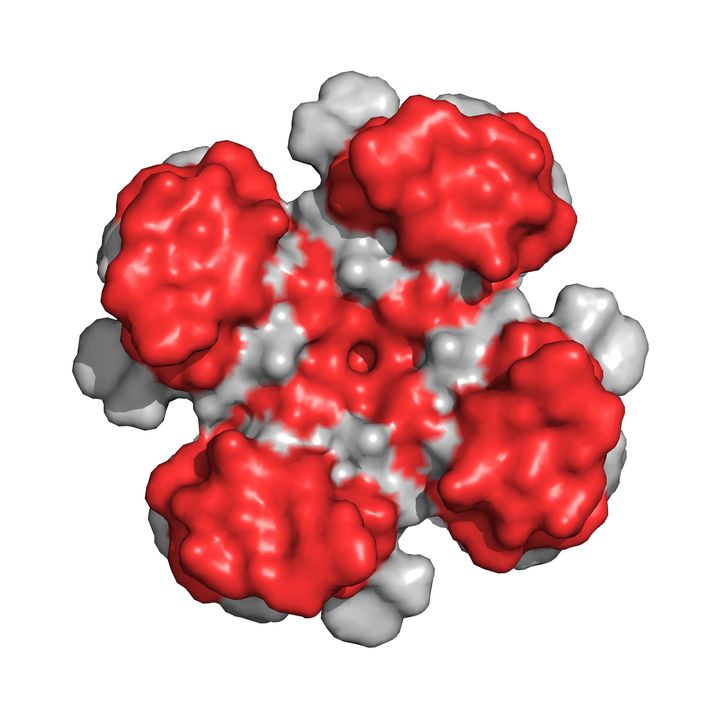
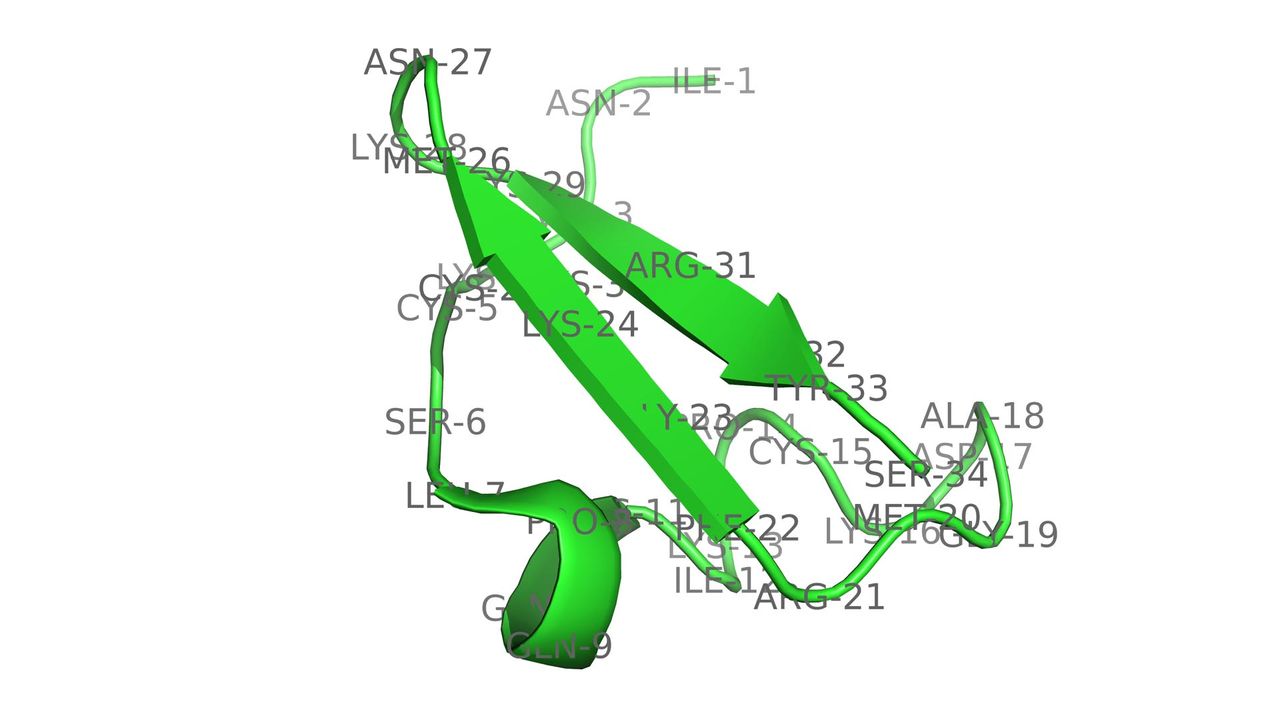
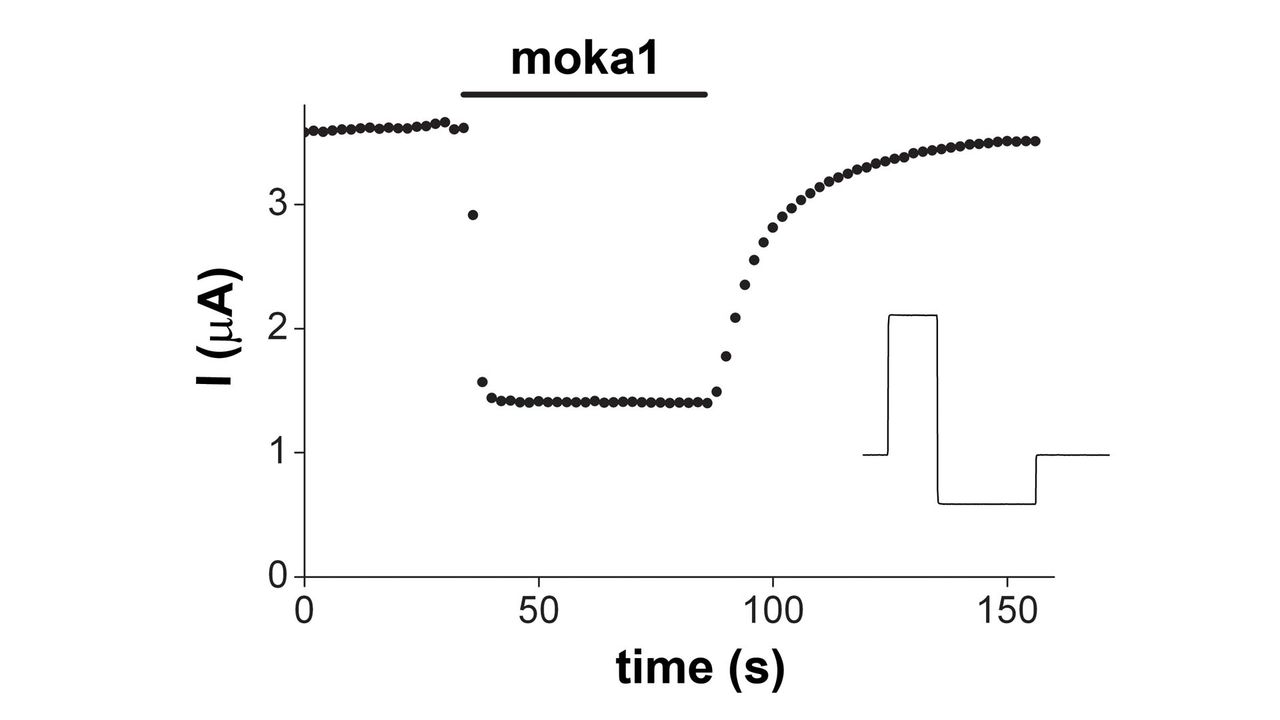
Designer Toxins are peptide ligands with custom biological properties that act on a wide variety of ion channels, other cell-surface receptors, and enzymatic pathways ("receptors"). Designer Toxins are produced by a proprietary technology platform which generate thousands to millions of novel combinatorial variants of evolution-tested, target-biased animal toxins and a screening platform to isolate those hits that are active on the pharmaceutical targets of interest in their native cellular milieu or in a purified form. Designer Toxins can be employed to identify new hits or leads for drug development, to optimize known ligands (e.g., selectivity, mechanism of action, pharmacokinetics), and to validate therapeutic target selection. Public domain examples produced by Designer Toxins are high-affinity, selective ligands for Kv1.3 and KcsA K+ channels.
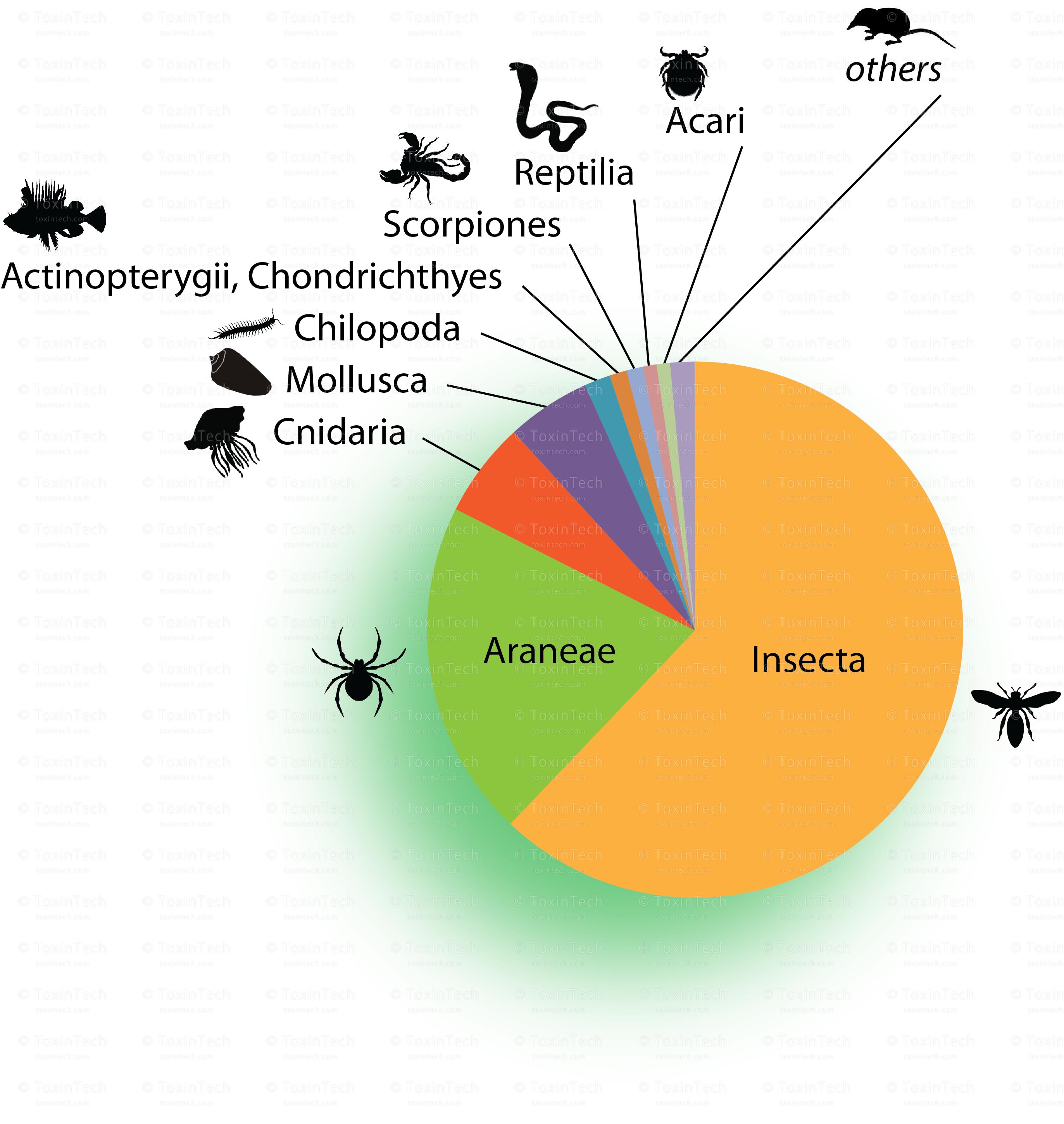
Today, animal venom toxins are the source of a number of major medications, including first-in-class agents, with indications for a diverse range of diseases from cardiovascular disorders to metabolic and to chronic pain. Additionally venom toxins are the source of a number of diagnostic and drug-cosmetic agents.